Healgen Scientific COVID-19/Flu A&B Ag Combo Rapid Test Cassette (Swab)
Healgen Scientific COVID-19/Flu A&B Ag Combo Rapid Test Cassette (Swab)
Couldn't load pickup availability
The Healgen® COVID-19/Flu A&B Antigen Combo Rapid Test Cassette is a quick and reliable diagnostic tool designed to detect and differentiate SARS-CoV-2, Influenza A, and Influenza B from a single nasal swab specimen. Intended for use within the first five days of symptom onset, this CLIA-waived test empowers healthcare providers to deliver timely care and help prevent the spread of infection. Results are available in just 15 minutes, supporting fast clinical decision-making at the point of care.
Key Features
-
Triple Detection: Identifies COVID-19, Influenza A, and Influenza B antigens with one nasal swab.
-
Rapid Results: Delivers accurate results in as little as 15 minutes.
-
CLIA Waived: Approved for use in point-of-care settings by trained healthcare professionals.
-
Reliable Performance: Test results demonstrate strong accuracy with positive percent agreement rates of 90.2% (COVID-19), 90.0% (Flu A), and 91.2% (Flu B).
-
Extended Shelf Life: Stable up to 16 months at room temperature (2–30°C / 36–86°F), making it convenient for seasonal preparedness.
-
Quality Controls Available: Optional external quality controls to maintain testing consistency.
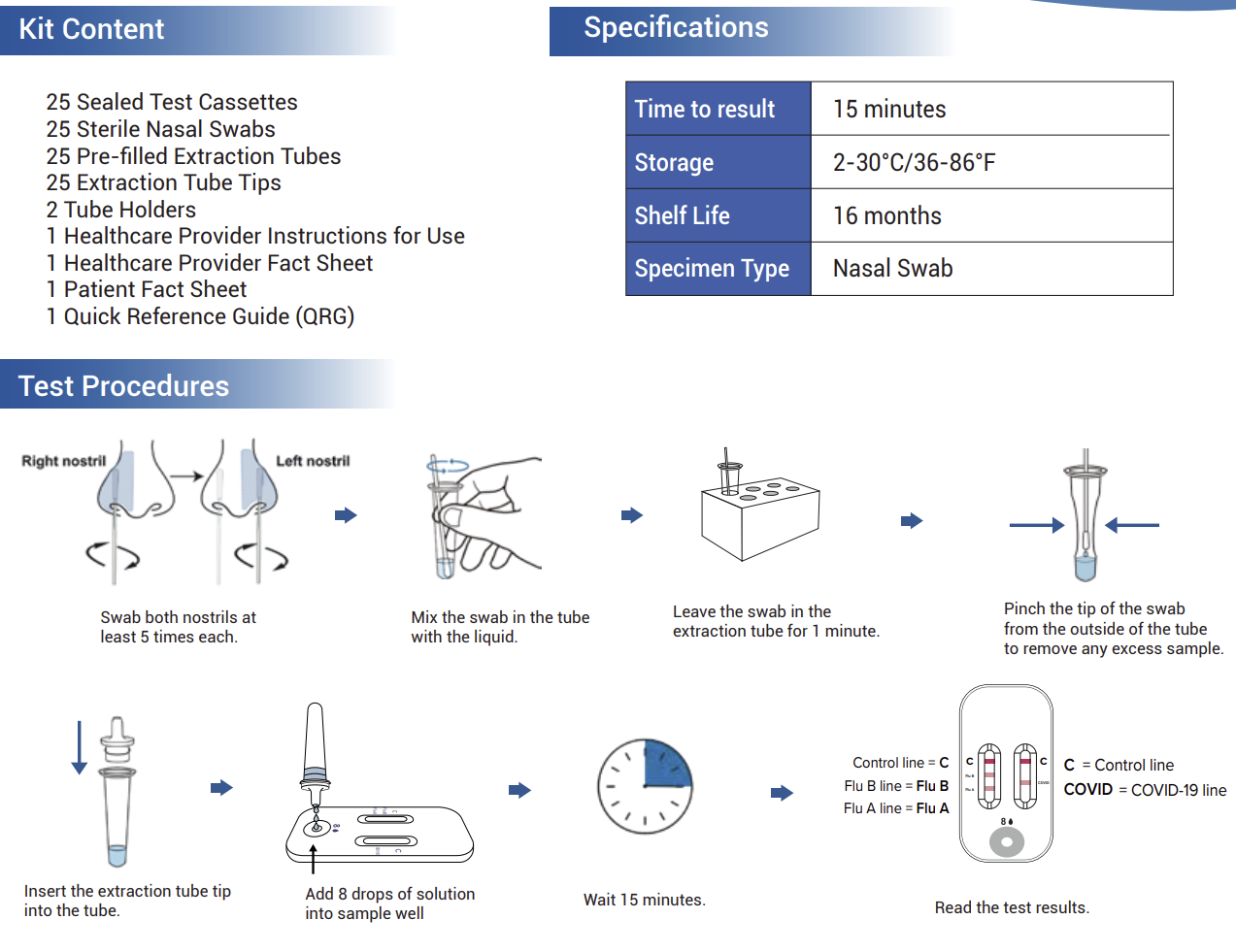
Product Specifications
-
Test Type: Lateral flow immunochromatographic assay
-
Time to Results: 15 minutes
Box Contents (25 Tests)
-
25 Individually Sealed Test Cassettes
-
25 Sterile Nasal Swabs
-
25 Pre-filled Extraction Tubes
-
25 Extraction Tube Tips
-
2 Tube Holders
-
Instruction Sheet for Healthcare Providers
-
Fact Sheets for Providers & Patients
-
Quick Reference Guide (QRG)
This product has not been FDA cleared or approved, but it has been authorized by the FDA under an Emergency Use Authorization (EUA) for use by authorized laboratories. It is only authorized for the detection of SARS-CoV-2, Influenza A, and Influenza B antigens and should not be used for detection of any other pathogens.
Licensing requirements may vary by state or locality. Please verify regulations prior to purchase.
Share